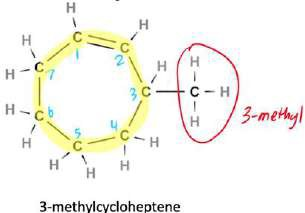
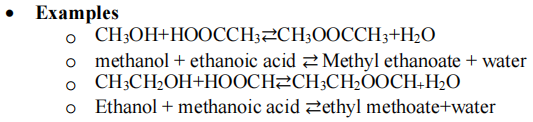Nomenclature
Inquiry question: How do we systematically name organic chemical compounds?
Students:
- Investigate the nomenclature of organic chemicals, up to C8, using IUPAC conventions, including simple methyl and ethyl branched chains, including: (ACSCH127)
-alkanes
-alkenes
-alkynes
- alcohols (primary, secondary and tertiary)
- aldehydes and ketones
-carboxylic acids
- amines and amides
- halogenated organic compounds
NOMENCLATURE:
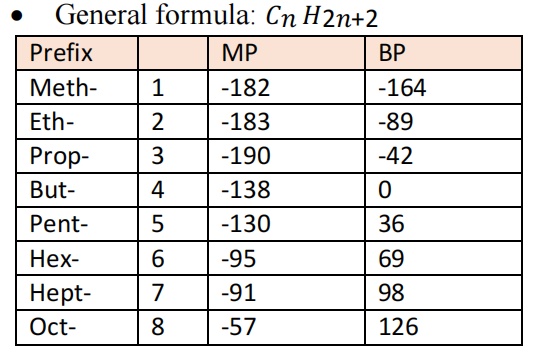
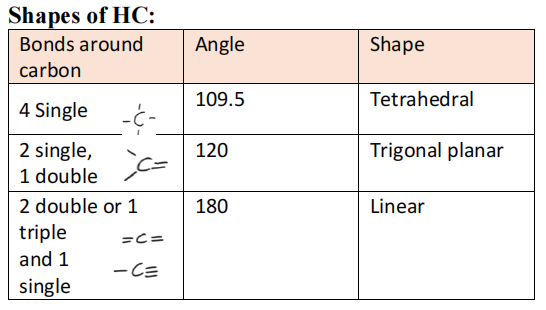
Hydrocarbons (HC) contain only hydrogen and carbon and can be classified as saturated or unsaturated. They are the main components constituents of natural gas and crude oil. Saturated (unreactive) HC contain single bonds whereas unsaturated (reactive) contains double or triple bonds. A homologous series is a family of compounds that is represented general molecular formula. A functional group of atoms giving a compound some characteristic physical and chemical property. They can be aromatic (having benzene rings) or aliphatic (everything else)
- As molecular mass increases, ignition temperature, BP, MP increases
-Gas → Liquid → Solid
- Colourless and Odourless
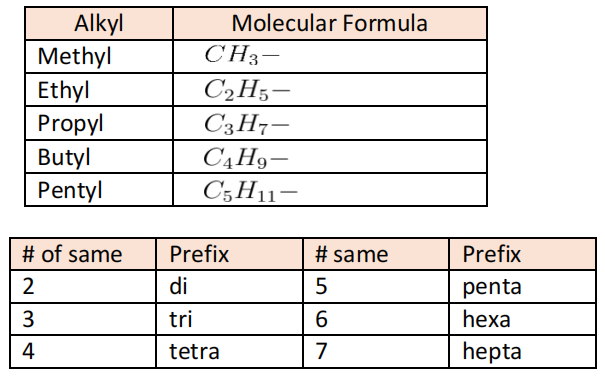
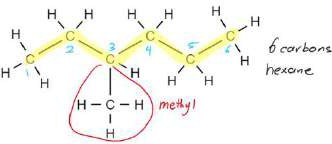
- Nomenclature: Find the longest chain, look for the branched alkanes and add the prefix alphabetically and add assign numbers to groups with lowest numbers.
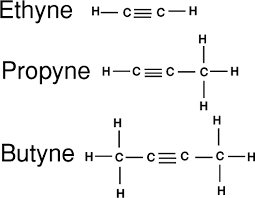
Alkenes:
- General formula CnH2n - 2
- Consists of double bonds. The more double bonds, the more reactive it
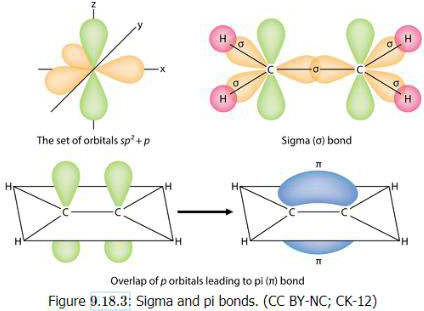
- Pi bonds make it more reactive and is formed by the overlap of orbitals in a side to side fashion.
- Sigma bonds formed from overlaps of orbitals in an end to end fashion.
- Sigma (σ) bonds are strong since they involve orbitals that overlap
- Pi (π) are weak since orbitals don’t overlap
- C=C has 1π bond and 1σ bond
- C≡C bond has 2π and 1σ bond
- It can accept more H atoms. It is necessary to assign a number to describe location of double bond.
- Flammable and volatile, strong odour, BP less than alkanes, insoluble and less dense than water.
- As molecular mass increases, ignition temperature, BP, MP increases
- Gas → Liquid → Solid
- Sigma bonds formed from overlaps of orbitals in an end to end fashion.
- Sigma (σ) bonds are strong since they involve orbitals that overlap
- Pi (π) are weak since orbitals don’t overlap
- C=C has 1π bond and 1σ bond
- C≡C bond has 2π and 1σ bond
- Volatile and flammable, no odour, insoluble and less dense in water.
- As molecular mass increases, ignition temperature, BP, MP increases
- Gas → Liquid → Solid
Cyclo HC
- Ring Structure and can be saturated or unsaturated.
- C-C → cycloalkanes
- C=C → cycloalkenes
- C≡C → cycloalkynes
- Aromatic HC è ring of C- atoms linked such that they have delocalised e-. E.G Benzene
- Has greater stability and usually unreactive
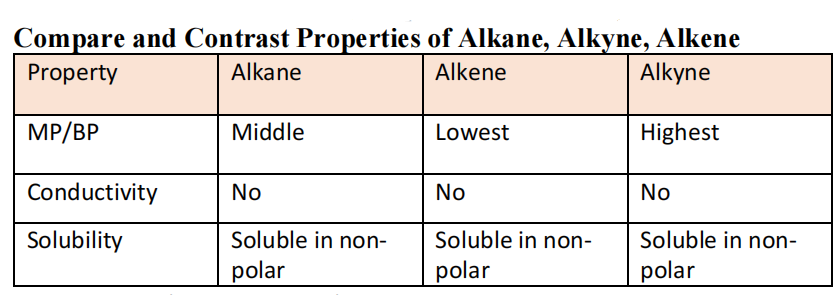
Properties of Alkanes/Alkenes/Alkynes
- Alkanes
- Non-polar è The C-H bonds have dipole-dipole as strongest intermolecular forces but the molecules general shape causes it to be non-polar. Hence, the strongest intermolecular force is dispersion forces as it is formed from temporary e- distribution. Resultingly, it has low MP/BP as little energy is required to overcome the forces. Being non-polar, it is not soluble in polar substance like water and is a poor conductor of electricity. It is relatively stable and unreactive.
- Alkenes/Alkynes
- Non-polar with dispersion forces. They are unsaturated due to bond type and hence are more reactive as they can form extra bonds. Similar physical properties to alkanes.
Alcohols
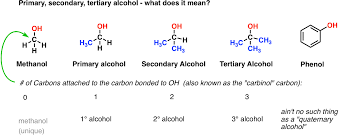
- Contains hydroxyl functional group.
- Naming: Identify longest chain and use corresponding prefix, write alkane without ‘e’ and put ‘ol’, state the position of alcohol before the suffix and then name substituents.
- Note: The OH group takes precedence over HC side chains, halogen substituents, double and triple bonds.
- If there is a double bond and hydroxyl group, the location of double bond goes before prefix (4-Penten-2-ol). If something else takes more precedence than alcohol, it is named using hydroxyl.
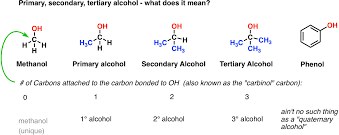
- Primary: One carbon attached to OH
- Secondary: Two carbon attached to OH
- Tertiary: Three carbons attached to OH
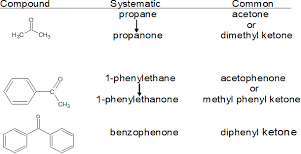
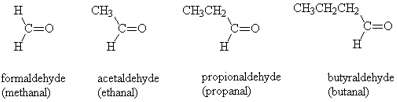
Aldehydes and Ketones
- Considered as carbonyl compounds (carbon atom double bonded with oxygen)
- Carbonyl located at end of the chain
- Naming: Identify longest chain with -CHO, then for an alkane replace ‘e’ with ‘al’ and then use normal naming convention.
- Ketoneo Located anywhere but on the end chains.
- Naming: Identify longest chain with -CO, then for an alkane replace ‘e’ with ‘one’, identify lowest position number and then use normal naming convention
Carboxylic acids
- Carboxyl functional group (COOH)
- Found at the ends of chains.
- Naming: Identify longest chain with COOH, delete the ‘e’ of alkane and replace it with ‘oic acid’ and then use normal naming convention.
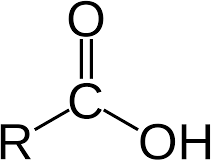
Amines and amides
- Amines:
- Amine functional group (NH2)
- Primary: has one alkyl group
- Secondary: has 2 alkyl groups on nitrogen
- Tertiary: has 3 alkyl groups on nitrogen
- Naming: Identify longest chain with N, delete ‘e’ of alkane and replace with ‘amine’. For primary, make N have lowest position number (propan- 2-amine). Else, name other alkyl groups in alphabetical order, and give them position N (N-methyl, N,N-dimethyl)
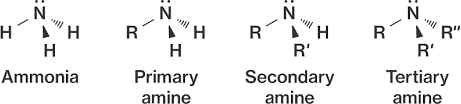
- Amides:
- Amide functional group (CO-NH2)
- Just like amines, amides are primary, secondary and tertiary.
- Naming: Identify longest chain with connected to N, delete ‘e’ of an alkane and insert ‘amide’ and give it the lowest position number, then name other alkyl groups connect to N in alphabetical order and then normal naming conventions.
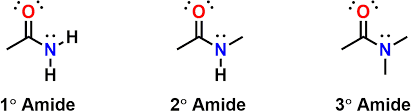
Naming priority

ISOMERS:
Compounds with same molecular formula but a different structural arrangement. It affects chemical and physical properties of compounds. There are different types of isomerism: structural, geometric and optical.

Chain isomer
- Isomers with different placement of substituents on main chain
- Rearrangement of chains into different branches.
- Longer the chain = more isomers
- Pentane C5H12 has 3 chain isomers
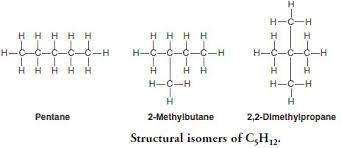
Position isomers
- Basic carbon skeleton remains unchanged but important groups are moved around.
- These are structural isomers with different functional groups.
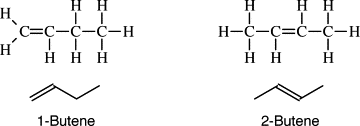
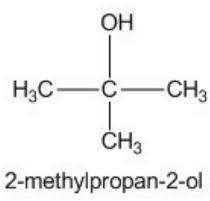
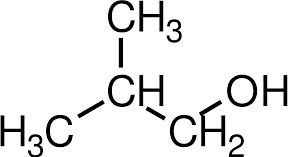
Functional group isomer
- Molecules with same molecular formula but different functional group and structural formula.
- The isomer contains different functional group
- Eg: C2H6O can be ethanol or dimethyl ether
- Eg: C3H6O can be propanol or propanone
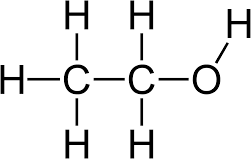
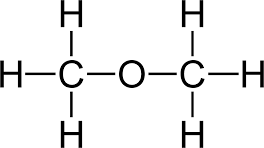
HYDROCARBONS:
Models are a simplified version of a part of an organic compound. Ball and stick models are usually used. Organic compounds can be described mainly in 5 ways. Benefits of modelling: Beneficial in illustrating differences between each homologous group by showing single, double and triple bonds that are present, provide a better understanding of isomers and visualise the processes which cannot be seen. Limitations are: There are no sticks in reality so its an inaccurate representation of the molecules shape and it is obviously not to scale.
Investigation
Aim: To investigate the structures of hydrocarbons through modelling.
Method:
Construct ethane, ethene and ethyne with black balls representing carbon atoms and smaller white balls representing hydrogen atoms.
Construct simple alkanes, alkynes and alkenes.
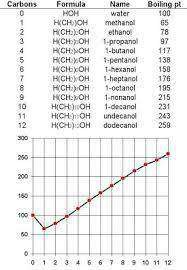
Homologous Series
- Series of compounds with same functional group
- Many physical properties such as BP gradually increase with increasing molecular mass
- Five main characteristics
Same series represented by general formula
Successive members differ by CH2
- Physical properties change regularly with increasing number of carbons which has extra dispersion forces creating stronger intermolecular forces
Similar chemical properties
Prepared using same method
- Ethanol, Propanol and Butanol prepared by hydration of alkanes.
Explain the properties
- BP/MP
Molecules with a variety of intermolecular forces cannot move easily and require more kinetic energy to break these bonds to form liquids/gases. So stronger the intermolecular forces, higher the MP/BP
Alkanes: longer chain è higher Molecular mass è greater number of electrons so higher dispersion forces → higher MP/BP
Non-branched chains have higher MP/BP than branched as branches reduce contact area resulting in weaker dispersion forces. As chain increases, surface area increases which increases the intermolecular forces between each molecule.
straight chain alkanes have greater surface area than branched alkanes
Alkenes: longer chain/Fewer branches increases MP/BP However it is lower than alkanes as they will have 2 fewer electrons for each double bond present as it decreases molar mass.
Alkynes: have the highest MP/BP due to the triple bond, fewer H atoms allows alkyne molecules to lie closer together, creating stronger dispersion forces.
- Volatility
- Polarity
Intra/Intermolecular forces
- Polarity
Non-polar, C-H bond weakly polar due to differences in electronegativity, however due to the symmetry of C-H bonds, any dipoles are balanced out so alkanes cannot mix with water.
- MP/BP
Low MP/BP due to weak intermolecular forces. Methane, ethane, propane and butane gas at room temp
Longer chain = stronger intermolecular force = more energy to break them
- Electrical Conductivity
- Viscosity
- Saturation
- Maximum number of hydrogen atoms intramolecularly bonded with carbon. They are better fuels as they release more energy in combustion reaction.
Safety with hydrocarbons
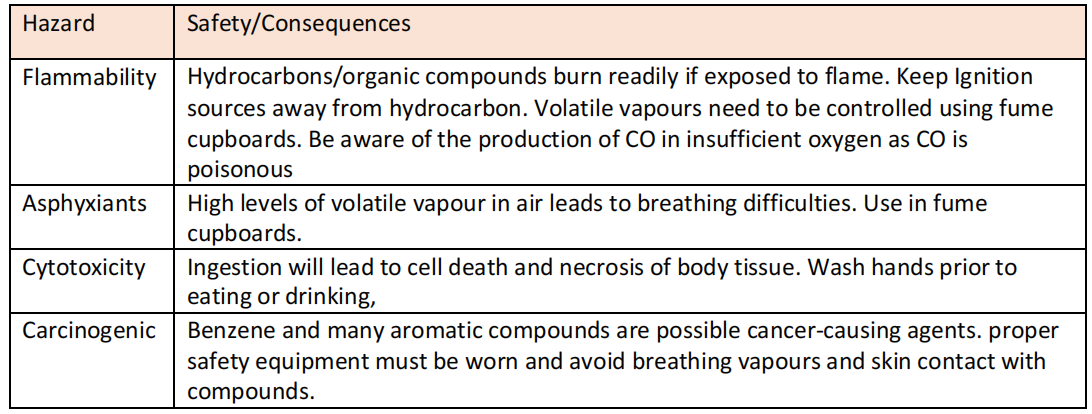
Disposal
Should not be disposed in sewerage as they are cytotoxic, they can kill organisms that are part of treatment at sewage treatment plant.
Should be disposed in suitable container which is collected by a license waste disposal contractor.
How are hydrocarbons found
- Majority comes from mining petroleum
- Petroleum is a fossil fuel
- Millions of years ago algae and plants died and sank to the bottom of the sea floor. High pressure and temperature transformed the corpses into fossil fuels.
Environmental implications
- Fossil fuels extracted by mining for coal or drilling for natural gas
- Mining causes:
- Erosion, formation of sinkholes, contamination to soil, ground water, surface water and deforestation
- Deforestation, erosion, long term exposure causes cancer, production of methane which is a more effective greenhouse gas than carbon dioxide and extensive land degradation.
- Increasing effect of global warming, air pollution, production of nitrogen oxides which contribute to acid rain, production of sulphur dioxide creating acid rain, widespread production of polymer plastic pollutes waterways oceans and lands.
Economic implications
We are dependent on fossil fuel for energy, viral growth to economy, runs agricultural industry and power generation, oil prices are one of the main causes of inflation as we are dependent on it, natural disasters that damage oil storages causes oil prices to rise and as more technologies run on renewable energy, tech which is dependent on fossil fuels will become outdated and no longer be produced.
Sociocultural implications
Health
Social
Industrial
Tourism impacted by climate change, great barrier reefs corals are dying and sea levels rising impacts small island states.
Climate change causes a shift in precipitation, temperature and agricultural zones, making it harder to grow crops
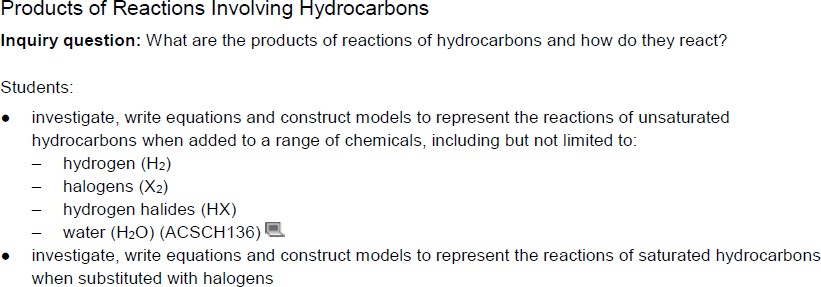
Products of reactions involving hydrocarbons
Unsaturated hydrocarbons are highly reactive due to the presence of the double/triple bond. The
double and triple bonds are weaker, easier to break and results in higher reactivity of molecules containing these bonds.
- Alkanes are fairly unreactive unless exposed to heat/light. They can undergo combustion reactions.
- In the presence of light or in high temperatures (800 degrees Celsius), alkanes react with halogens to form alkyl halides.
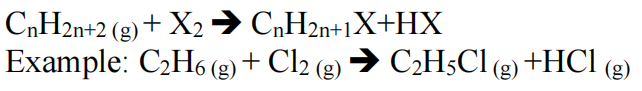
- Hydrogenation
- Adding hydrogen to alkenes/alkynes
- Since the bond is weaker than single bond, it can be broken easily and is reactive
- Addition of hydrogen creates single bond with additional hydrogen added to structure.
- Done in the presence of a catalyst.
- This reaction is quite slow
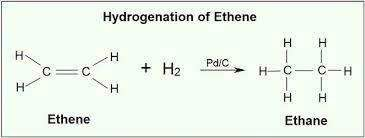
- Hydrogen molecule reacts with the metal catalyst, breaking one of its bonds between the 2 hydrogen atoms, forming 2 weak metal hydrogen bonds. The alkene molecule reacts with catalyst, breaking the double bond. Metal catalyst causes hydrogen atom to join with carbon atom.
- Only uses so reaction does not occur in high temperatures. The reaction is exothermic, and temperature should not be too high.
- Used in manufacture of margarine as raw fats and oils used are unsaturated. After saturating it, only side affect is that it creates trans-fats.
- Used in petroleum industry, it is used to convert alkenes and aromatics into saturated alkanes which is less toxic and reactive
Halogenation
- Adding halogen across double/triple bond
- Reacts readily with halogens so no catalyst required
- Addition of fluorine reacts explosively with alkenes and produces carbon and hydrogen fluoride gas
- Instead of slow should be fast
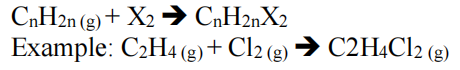




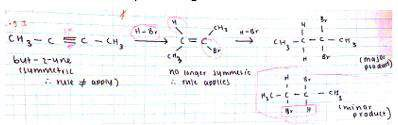
- Products that don’t will be formed at lower concentrations (minor product)
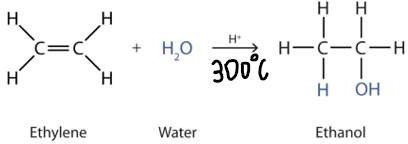
Hydration
- Adding water to alkene produces alkanol as we consider water to have H-OH structure. It requires a catalyst of dilute sulfuric acid.
- When water is added across a double bond, one of the hydrogens form the water molecule attaches to one carbon originally bonded in the double bond
- Follow Markovnikov rule
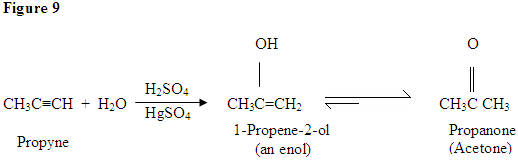
- Hydration of alkynes is catalysed by mercury (II) compounds and sulfuric acid
- Addition of water to alkyne produces ketone
- An exception is hydration of ethyne as that only produces ethanol
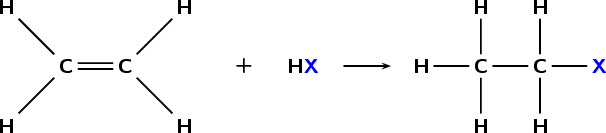
Alcohols
They have a general formula of CnH2n+1OH and show isomerism in the same way as alkanes, alkenes and alkynes. Primary: one carbon atom is bonded to carbon atom attached to OH Secondary: two carbon atoms bonded to carbon atom attached to OH Tertiary: three carbon atoms bonded to carbon atom attached to OH.

Properties
- Boiling point
- Alkanes/ens/ynes have relatively low MP/BP as they only form dispersion forces.
Compared to alcohols of the same number of carbons, they have a high BP. This is
because of the OH (Hydrogen bonding) to occur between alcohol molecules. This is
much stronger than dispersion forces. - Longer the chain = higher BP
- Solubility
- Relies on the balance between polar functional group and non-polar hydrocarbon chain.
- Small alcohols completely soluble in water because they easily form hydrogen bonds in water
- When small alcohol molecules placed in water, alcohol-alcohol and water-water H bonds must be broken to be mixed. Energy released by the formation of new alcohol-water H bonds approximates the energy needed to break the original H bonds, allowing them to be mixed.
- For C<4, effect of the polar hydroxyl group outweighs effect of non-polar hydrocarbon chain hence becoming soluble. Else, most of the molecule is a hydrocarbon tail which outweighs the polar hydroxyl forcing the water and alcohol not to mix.
- For big carbons to be soluble in water, you must have multiple hydroxy groups.
- Viscosity
- Property of fluid that resits force tending to cause the fluid to flow
- This increases in size for alcohol as molecule increases due to the increase in strength of the intermolecular forces which hold the molecules more firmly
- Flammability
- Decreases as the size and mass increases
- Combustion breaks the covalent bonds of the molecules, so as size/mass increases, there are more covalent bonds to break in order to burn that molecule, so more energy is required to break all of them.
- Polarity
- Amide>acid>acid>alcohol>ketone≈aldehyde<amine<ester<ether<alkane
- Ranked third due to its hydrogen bonding capabilities and presence of 1 oxygen
- Carboxylic acid is more polar as it has 2 oxygen molecules
Bonding in alcohol
- Hydrogen bonding
- Hydrogen atom on one alcohol molecule forms hydrogen bonds with oxygen atom of another molecule. Ince Hydrogen bonds are extremely strong, more energy is need to break these bonds hence it has a higher BP/MP than equivalent alkane
- Dipole-dipole
- Due to the electronegativity difference between oxygen and hydrogen atoms which results in a dipole. The C-O bond is also a polar covalent bond because Oxygen is much more electronegative than carbon so it pulls electrons away from the carbon which results in another dipole forming between carbon and oxygen atoms
- Dispersion forces
- The non-polar hydrogen tail experiences dispersion forces. As the tail gets longer, the affect of the hydrogen bond gets smaller relative to the size of the molecule so there will no longer be a significant difference between boiling points of alcohols and alkanes.
- Intramolecular forces
Investigation enthalpy
Aim: To determine and compare the heat of combustion per gram and per mole of 3 alkanols using a calorimeter Safety issues: Conduct in well-ventilated area since incomplete combustion may occur Methodology:
- Weigh empty aluminium can with an electronic balance
- Fill can with 100ml water
- Re-weigh can to find mass of water
- Measure the initial temperature with a thermometer
- Weigh the spirit burner containing the alkanol.
- Heat the can for 10 minutes for all three alkanols
- Record waters final temperature
- Extinguish the burner and reweigh it to calculate moles
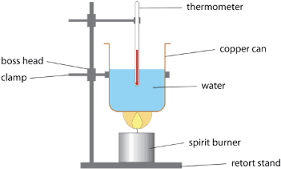
Results:
Heat released from combustion calculated by Q=mC∆T
Heat of combustion calculated by ∆HC=-Q/nfuel
Methanol has lowest heat of combustion, then ethanol then propan-1-ol
Justification of method:
- Aluminium can used as it’s a better thermal conductor than glass and allows for a greater transfer of heat
- The 3 alkanols chosen most likely to undergo complete combustion
Limitations and improvements
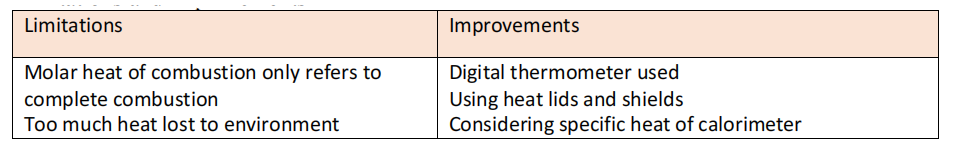
Specific heat of aluminium was not considered
Reactions of alcohols
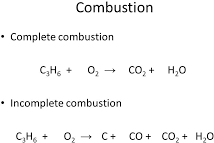
- Combustion
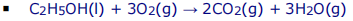
- Incomplete combustion: Insufficient oxygen è produces CO, C and water
- Dangerous because CO prevents oxygen reaching organs and hinders cell respiration and if carbon soot is inhaled, it coats the lungs decreasing its ability to take in oxygen.
- Dehydration
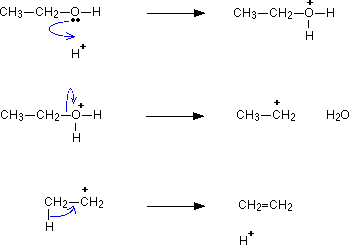
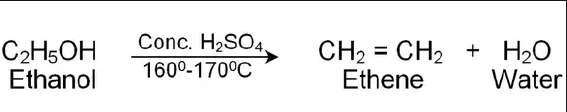
- Removal of OH plus hydrogen from adjacent hydrogen. Results in the formation of alkene and water
- Must be in presence of a strong acid (sulfuric/phosphoric acid)
- For more complex alcohols, more than 1 alkene is produced (Butan-2-ol)
- If OH is removed, then there are 2 possible hydrogens that can be removed as well.
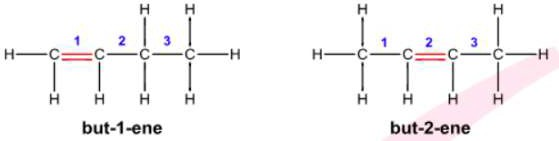
- The products become but-1-ene and but-2-ene. Also, but-2-ene has two geometric
isomers, cis-but-2-ene and trans-but-2-ene. - However, its randomly made.
Temperature range
- Primary alcohols: 170 to 180 degrees
- Secondary alcohols: 100 to 140 degrees
- Tertiary alcohols: 25 to 80 degrees
Steps:
- Acid donates H to OH, water is released from molecule and
them water takes another H from the molecule and the
remaining charged carbons form a double bond
- Substitution with HX
- Alcohols react with hydrogen halides by substituting the halide for the OH group and creating water.
- Reactivity order: Tertiary>secondary>primary>methyl
- Hydrogen halide reactivity order: HI>HBr>HCl>HF
Steps:
H-X ionises into H+ and X- , then the H+ attaches to the OH group and the
group is released to form water then the X replaces OH
Butan-2-ol + HBr → 2-bromobutane+water
C4H9OH(aq)+HBr(aq)èC4H9Br(aq)+H2o(l)
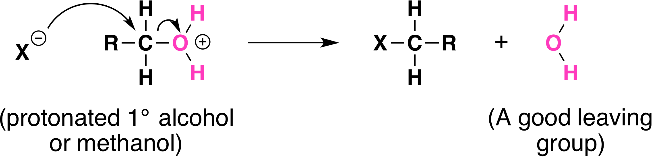
- Oxidation
- Can be oxidised into aldehyde, carboxylic acid or ketones
- Primary
- Oxidised to form aldehyde and further oxidation creates carboxylic acid
- To produce aldehyde: excess alcohol used compared to oxidising agent to prevent aldehyde from being oxidised as it is formed. The aldehyde also need to be removed from the reaction vessel quickly to minimise with the oxidiser
- To produce acid: Excess oxidiser used such as alkaline potassium permanganate (KMnO4) or potassium dichromate (K2Cr2O7)
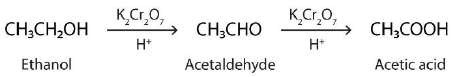
- Secondary
- Oxidised into Ketone
- Cannot oxidise into acid as there are no free hydrogens on carbonyl carbon to create hydroxyl group
- Produces water as well
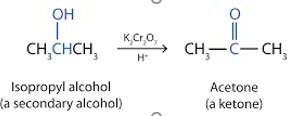
- Tertiary
- Cannot be oxidised into anything
- Oxidation removes hydrogen from OH and a hydrogen from the carbon it is attached to.
- In this case, the carbon has no hydrogens
- When dichromate ions used in oxidation, its orange colour changes to green
- Under acidic conditions, permanganate ions go from purple to colourless
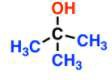
Fermentation
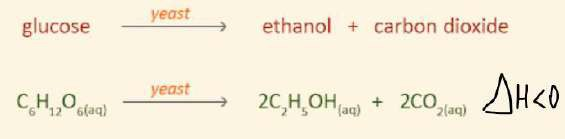
- Ethanol concentrations past 15% by volume, yeast cannot survive
- Once fermentation finished, ethanol is separated from mixture by fractional distillation to convert ethanol into higher concentrations (95-100%)
- In anaerobic conditions, yeast is fermented into ethanol and carbon dioxide
Structures of haloalkanes
- Haloalkanes can be primary, secondary or tertiary
- Primary: one carbon bonded to carbon with halogen attached
- Secondary: 2 carbons bonded to carbon with halogen attached
- Tertiary: 3 carbons bonded to carbon with halogen attached
- Tertiary>secondary>primary
Substitution with haloalkanes
- Addition of water to haloalkane reacts to form a H-X and alcohol
- Nucleophile: electron rich species donates electron pair to electrophile to form a bond. Attracted to regions of positive charge
- Electrophile: positively charged or neutral species which is attracted to regions of negative charge
- Alkyl halides undergo a nucleophilic substitution reaction with water or OH- to form alcohol
- Nucleophilic water/OH- will react with carbon attached to the halogen group, forcing the halogen to leave molecule
- Carbon halogen bond easier to break than carbon hydrogen bond
- Exception: Carbon-fluorine bond is an exception
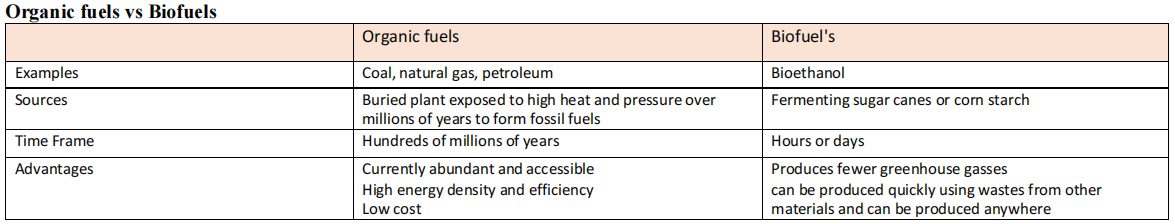

Ethanol
Ethanol has great potential to be used as a renewable alternative fuel, however, its widespread use is currently limited due to its cost barriers as the cost of producing ethanol is higher than petrol.
Organic acids and bases

Bonding in carbonyl group
- Oxygen is far more electronegative than carbon and so has a strong tendency to pull electrons in a carbon-oxygen bond towards itself making it highly polar.
- Aldehyde has hydrogen atom attached to this group making it easy to oxidise. Since ketones don’t have this, they are resistant to oxidation except when exposed to Powerful oxidising agents which break C-O bonds
BP and MP of carbonyl group
- Has higher MP and BP than hydrocarbons of similar molecular weight
- This is due to the dipole-dipole forces formed between aldehydes and ketones.
- Lower MP and BP than alcohols due to hydrogen bonding
Solubility of carbonyl group
- Highly polar, forms an attraction to highly polar water making them more soluble than hydrocarbons but less soluble than alcohols
- Small aldehydes and ketones soluble in water
- Longer the chain, less solubility (C<4 are soluble as they form H-bonds with water)
BP and MP
- BP and MP of amines
- Very polar due to N bond. Primary and secondary amines can form hydrogen bonds however its weaker than alcohols
- BP and MP lower than alcohols
- BP and MP of amides
- Primary and secondary also polar due to N-H and C=O bonds
- Tertiary has no N-H
- Primary and secondary can form hydrogen bonds between molecules and form dimers
- Very high MP and BP
- SECONDARY>PRIMARY>TERTIARY
- As chain increases, odour becomes stronger
- Solubility of amines and amides
- Primary and secondary amines and amides of C<6 are soluble in water because they easily form H-bonds with water molecules. However, as the hydrocarbon tails get longer, they force themselves between molecules and break H-bonds, making amines and amides longer lengths insoluble in water
- Tertiary amines and amides are soluble but less soluble than primary and secondary
- Carboxylic acid properties
- Can form a range of intermolecular forces
- Hydrocarbon chain forms dispersion forces, C=O forms dipole-dipole and -OH forms hydrogen bonding
- Increases as chain increases.
- Higher than corresponding alkane and alcohol due to larger dipole-dipole forces and formations of dimers (a molecule or molecular complex consisting of two identical molecules linked together)
- Small acids like methanolic acid and ethanoic acid are soluble in water, however for larger acids, the non-polar tail prevents the acids from being soluble. Rather they line up at the surface and is called surfactants
Intermolecular forces
- Amines: strongest bonds are hydrogen bonding and dipole-dipole making it polar
- Amides: strongest bonds are hydrogen bonding and dipole-dipole giving it high MP and BP
- Carboxylic acids: strongest bonds are hydrogen bonding and dipole-dipole increasing MP and BP. Dispersion forces of an increasing chain increase MP and BP.
- Amides/Amines: strongest force is dispersion and as chain increases, solubility decreases due to hydrocarbon tail being non-polar
Intramolecular forces
- Carboxylic acid/Amides/Amines: has non-polar covalent bonds (C-H produce this and are not good conductors of electricity as they can’t ionise in water). Has polar covalent bonds between elements: C=O, O-H, N-H contributing to dipole-dipole and hydrogen bonds
Esters
- Living Things store their energy as esters. Many flavours and odours of fruits are esters. An alcohol + carboxylic acid forms an equilibrium with ester and water.
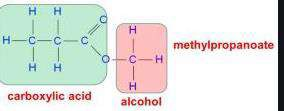
Naming esters
- First part is the alkyl group, methanol becomes methyl, propanol becomes propyl etc
- Second part is the carboxylic acid. Replace ‘lic acid’ with oate
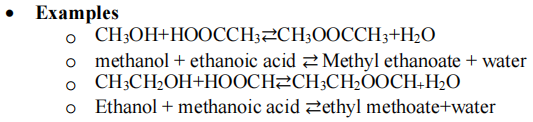
Properties
- Polar molecule, liquids at room temperature and BP much lower than carboxylic acids as main intermolecular forces are dipole- dipole which is weaker than hydrogen bonding
- Most esters are not soluble in water due to lack of hydrogen bonding and presence of hydrophobic alkyl group. Soluble organic solvents
- Esters are highly flammable and easily evaporated.
How to make ester
- Esterification: Reaction that forms an ester through the reaction between a carboxylic acid and alcohol molecule
- Formed when carboxylic group and hydroxyl group react releasing small water molecule and ester and is done using a reflux 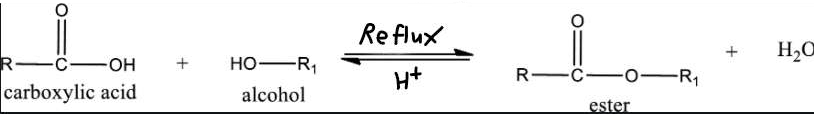
- Slow process so concentrated sulfuric acid acts as a catalyst for process to occur in slower amounts of time
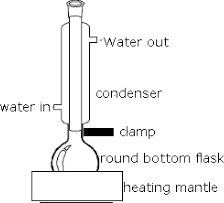
- Mixture heated to increase rate of reaction. However, using a Bunsen burner results in loss of mixture. The organic chemicals are volatile and will evaporate even when low heat is applied
- Reflux: Process of extended heating of mixture without it evaporating. As mixture is heated, volatile components evaporate and move to a vertical condenser where the gases are cooled so thy return to the flask.
- Heating mantle: since organic chemicals are highly flammable, naked flame not used. Heating mantle designed to provide heat without a flame
- To increase yield of ester, one reagent is added in excess as LCP predicts equilibrium will then shift to favour more ester
- After refluxing, you get organic components (Ester (not soluble in water), Carboxylic acid, alcohol) and inorganic compounds (water, sulfuric acid)
- Separating funnel is used.
- The entire mixture from the reaction flask is poured into a funnel and water is added.
- Organic layer is less dense and goes to the top.
- Inorganic layer removed.
- Sodium carbonate is added to remove any carboxylic acid as they will react to form carbon dioxide gas
- Distillation can be used to locate ester.
Prac: Synthesising octyl acetate
Aim: To synthesise the octyl acetate ester from octanol and acetic acid
Equipment:
- Flask
- Boiling chips
- Condenser tube and water hoses
- Bunsen burner and gas hose
- Separating funnel
- Beakers for waste
Method
- Transfer about 12mL of octanol and about 10mL of acetic acid into a flask
- Add several boiling chips and about 1Ml of concentrated sulfuric acid to the flask
- Attach a condenser tube to the flask and run water through the outer case of the tube
- Heat the flask with a Bunsen Burner such that mixture is evaporating and condensing but not escaping
- Allow mixture to cool and then transfer it to a separating funnel
- Add water to the separating funnel, swirl and allow it to stand
- Open the tap of the separating funnel and allow the aqueous layer to run out, leaving the octyl acetate remaining
- Open the tap of the separating funnel and allow aqueous layer to run out
Discussion
- Sulfuric acid used as a catalyst by acting as a dehydrating agent, forcing equilibrium to move to move to the right. Mixture is also boiled to speed reaction
- Reflex should be used as it improves safety.
Organic acids and bases
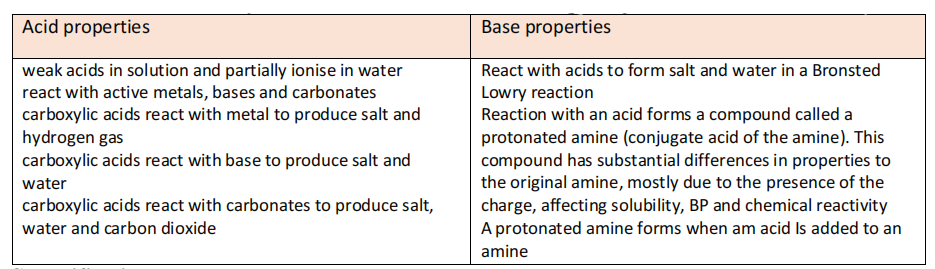
- Organic acid: most common is carboxylic acid. These are weak acids that do not completely ionise in water.
- Organic acids unusually acidic due to -OH bond. However, they vary in degree of ionisation due to 2 factors.
- Strength of the bond being broken (usually O-H bond)
- Stability of ion formed
- Acid strength: Sulfonic acid>carboxylic acid> phenol
- Sulfuric acid ionises to form a conjugate base containing extremely stable SO3- while carboxylic acids ionise to give carboxylic ion which is less stable and is a conjugate base
- Phenol least acidic as their conjugate (phenoxide ion) is less stable than carboxylic ion due to high electronegative C-O bond
- Examples: lactic acid, citric acid, acetic (ethanoic acid), formic (methanoic acid)
- Organic base: Usually based around nitrogen compounds. Amines most common type of organic bases. Exist in nature (DNA bases- Adenine, thymine, cytosine and guanine). Basic due to presence of a proton acceptor, usually nitrogen. E.g ammonia
- Strength depends on how easily lone pair picks up H+
- The stability of the ions formed: More the charge is spread around the molecule, more stable it is, more basic
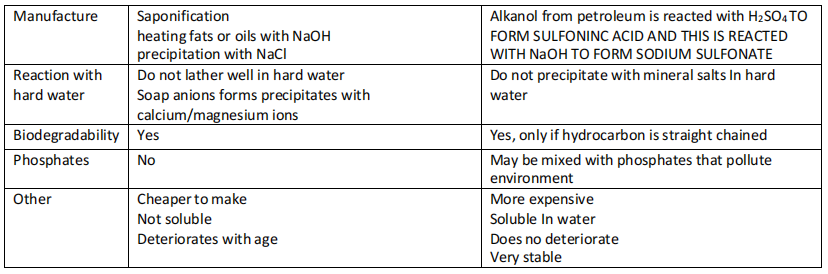
Saponification
- Hydrolysis of fatty esters. The saponification reaction typically refers to the reaction that is carried out by a strong base
- It is the reverse of esterification and is used to produce soaps from natural fats and oils (aq)
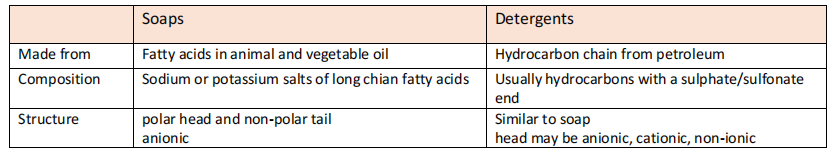
- Soaps: ionic compounds contains a long hydrocarbon tail and a negatively charged head that will dissociate with water. The tail is non-polar/organic tail/hydrophobic tail while head is polar head/hydrophilic head/inorganic head.
- Soaps are made through hydrolysis of fats in saponification reaction.
- Fats are triglyceride (molecules of 3 hydrocarbon chians containing 10-20 carbon atoms joined to a propane backbone by 3 ester bonds.
- Soap curdles are formed by heating fats.
- The curdles can be scraped off and dried to form solid soap. The remaining soap ions in solution are precipitated out by adding a solution of NaCl.
How soap works
- Long hydrophobic tail and hydrophilic head acts as surfactants which decrease surface tension of water. When soap is dissolved in water. Polar heads are stabilised and disrupt water’s H-bonding network, improving the ability of water to cling to dirt/oil particles.
- The hydrophilic head is attracted to water via H-bonding while hydrophobic tail interacts with the dirt/oil via dispersion forces.
- With agitation, soap molecules lift oil particles creating micelles
- Micelles are stabilised via the repulsion between negative polar heads. This stable mixture is an emulsion
- When a material is rinsed in water, the soapy emulsion of oil and water is washed off.
Emulsion
- Stable dispersion of small droplets of one liquid throughout another liquid (where 2 liquids don’t mix)
- Requires emulsifier to stabilise them
- Soap, water and oil form an emulsion
- Oil in water è small droplets of oil in water
- These dissolves and mix well with polar solvents and can be coloured with water soluble dyes to their high-water content.
- Water in oil è small droplets of water in oil
- Dissolves and mix well with organic solvents and can be coloured with oil soluble dyes die to their high oil content
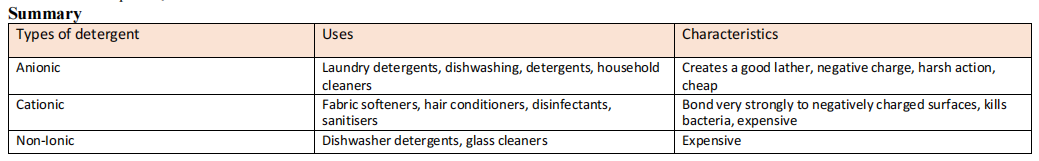
Detergent
- Man-made surfactant with a long hydrophobic tail and hydrophilic head
- Synthesised to be used instead of sap as soap is ineffective against hard water (contains high levels of calcium/magnesium ions)
- Works like soaps but is synthesised from fossil fuels
- Soap readily precipitates with the ions to form scum
Ca2+/Mg 2+ +2CH (CH ) COO- →Ca(CH (CH ) COO) (aq)3 2 16 (aq)3 2 162 (s)
- In highly acidic solutions, soap can also precipitate out to form another type of scum
H+ (aq)+CH3(CH2)16COO- →CH3(CH2)16COOH(s)
- Most detergents don’t form precipitates. However, anionic detergents may form soluble complexes with these ions which disrupts the cleaning action, not as bad as soaps though.
Anionic Detergents
- Containing a long hydrocarbon tail and a negatively charged head
- Most common is alkyl benzenesulfonates
- Excellent for cleaning natural fibres and glass
- Not used for personal hygiene as they remove too much oil form skin and hair
Cationic Detergents
- Contain a long hydrocarbon tail and a positively charged head
- Positively charged head is usually and alkylated quaternary ammonium group
- Cationic head binds strongly to negatively charged surfaces, which helps reduce static friction and tangling of fibres. Therefore, they are used in fabric softeners and hair conditioners
- Can be used in household disinfectants and sanitisers such as antiseptic soaps and mouthwashes
Non-ionic Detergents
- Contain long hydrocarbon tail and a polar segment marked by repeating ethoxy groups (CH3CH2O-) and OH head
- Produces much less foam than ionic detergents so they are used in dishwasher powders to prevent foam clogging up water jets.
- Used in paints, adhesives and cosmetics
Reaction pathways
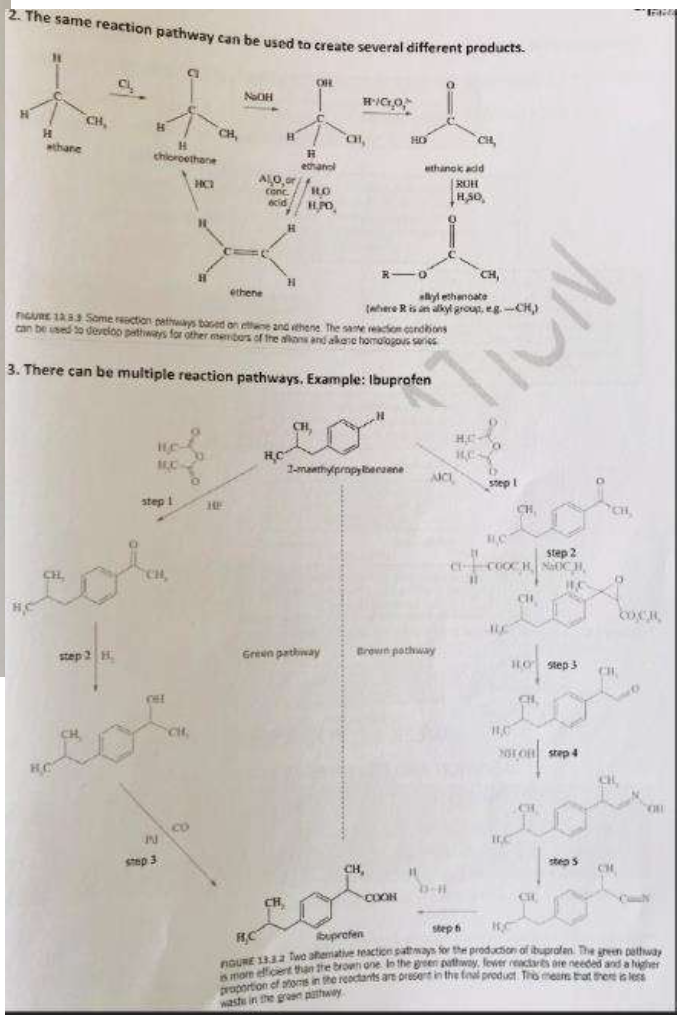
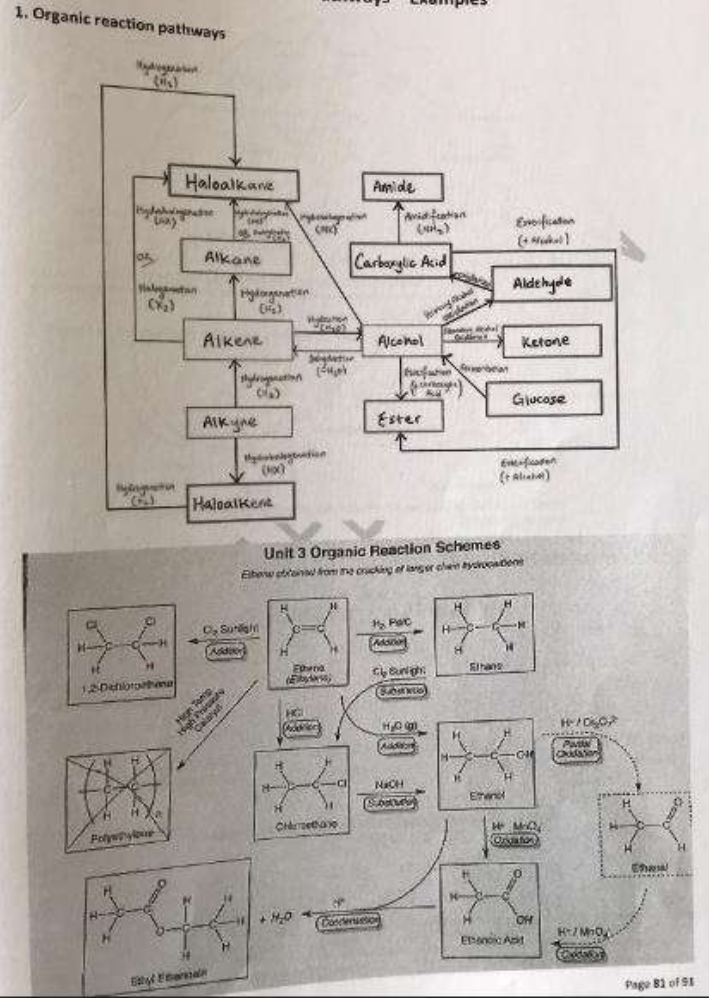
Polymers
Ethylene is the same substance as ethene. Polyethylene is the cheapest plastic. The weight of polyethylene produced each year is greater than the total of all other plastics. When petroleum undergoes fractional distillation, some fractions are in demand and of high economic value.
Other fractions consisting of larger molecules than in petrol and of low value can be passed over a heated catalyst that cracks the larger molecules into smaller molecules. A major by product of this cracking is ethylene.

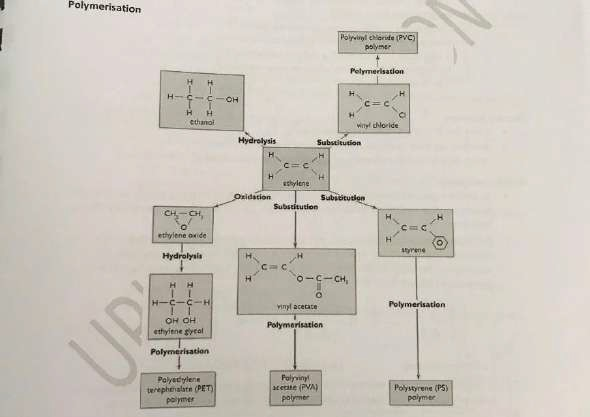
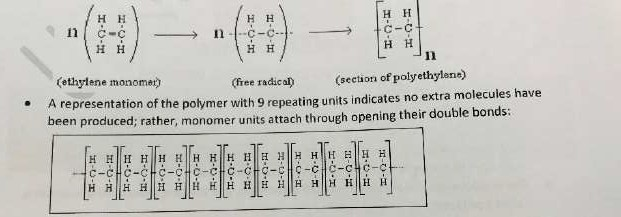
- This is the chemical reaction in which many small identical repeating unit molecules combine to form one long chain macromolecule
- The small identical molecules are called monomers and the long chain macromolecule is called polymers.
- Because of its reactive double bond, ethylene is able to undergo addition polymerisation è ethylene a monomer forms the polymer poly(ethylene)
- Polymers can be synthetic (plastics) or natural (silk, proteins, DNA)
- All plastics synthetic polymers, not all synthetic polymers are plastics
- Plastics are malleable, pliable and can be moulded into heat and pressure. Those that can be melted are called thermoplastic while those that can’t are called themoseting. Thermoplastic can be recyclable whereas thermosetting polymers can’t.
- Naming:
- Place poly Infront of monomer name
- Brackets are used when the monomer name is more than one word, or begins with a number
- Poly(monomer name) or poly(1-chloroethene)
Addition polymers
- An unsaturated monomer undergoes chain growth through the opening of its double bonds to form a saturated polymer molecule
- Forms only one product: The polymer molecule with no extra molecule is produced. Polyethylene is an addition polymer that forms by opening the double bond within each ethylene monomer unit to attach and form a polymer
- Initiation
- UV light is used to first break the bonds of weak halogen� halogen bonds to make radicals.
- Can also use any peroxides
- Propagation
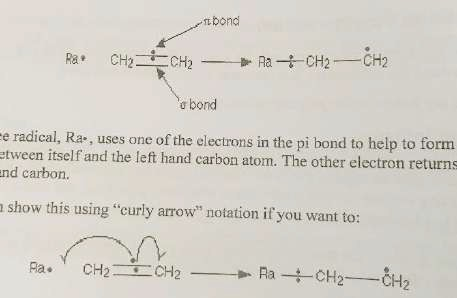
- Once a radical is made, it can react with stable molecules to form new radicals. One halogen radical then pulls away the hydrogen from the C-H bond, leaving a C- on the alkane. The C- pulls a halogen atom away from its molecule and terminates as a C-X bond but leaves behind another X- to continue process
- The free radical uses one of the electrons in the pi bond to help form a new bong between itself and the left-hand carbon atom. The other electron goes to the right-hand side. The new bond between the radical and carbon is stronger than the broken pi bond. You get more energy breaking the new bond. More energy release= more stability.
- Termination§
- Stops after another radical is added or 2 growing units add together
- Does not usually require a catalyst
Polymers of Ethene compounds
- Structure
- Repeating units of ethylene monomers
- Formula: n(CH2-CH2)
- Also called polyethene and is widely used plastic in the world
- LDPE (low density PE)
- High branching and less packed together= less dispersion forces. Impermeable to eater, thermoplastic, high flexibility, low melting point, high ductility, and low tensile strength
- HDPE (high density PE)
- Low branching and more closely packed, impermeable to water, thermoplastic, strong and stiff, highly crystalline structure and higher MP than LDPE. Ideal for rubbish bins, cutting board as its non-reactive
- LLDPE (linear low-density PE)
- Lots of short chain branching, at equal density and thickness, LLDPE has higher impact strength, tensile strength, puncture resistance and elongation than LDPE, used in high performance garbage bags, ice bags.
- Structure
- Repeating units of vinyl chloride monomers
- Formula: n(CH2-CHCl)
- Also called polychloroethene
- Properties
- Impermeable to water, thermoplastic, very hard, rigid and brittle. C-Cl is vulnerable to UV decomposition
- Additives are added to alter pure PVC properties: often softened to increase distance between polymer chain and UV to prevent quick decomposition. Used in drainage pipes (chemically modified), credit card (pure), garden hoses (softened)
-To design a polymer with specific properties, more than one monomer can be used.
- Combining a butadiene monomer with a styrene monomer improves the strength of the polymer
• Polystyrene
- Structure
- Repeating units of styrene molecule. Its an ethylene molecule with one of its hydrogen atoms replaced by benzene ring.
- Formula: n(CH2-CHC6H5)
- Properties
- Crystalline Polystyrene
- Impermeable to water, thermoplastic, hard and rigid. Used in screwdriver handles, kitchen door handles.
- Expanded Polystyrene
- formed by blowing gas into liquid polystyrene
- Lightweight and excellent thermal conductor, excellent shock absorber.
- Used in Styrofoam cups, surfboards, eskies
- Polytetrafluorethylene
- Structure
- Repeating units of terfluorene monomers
- Formula: N(C2F4)
- Properties
- HYDROPHOBIC, NON-REACTIVE DUE TO STRENGTH OF c-f BONDS, THERMOPLASTIC, HIGH MP, STRONG BUT FLEXIBLE
- Used in cookware, pipelines.
- Deteriorates at 260 degrees Celsius

Condensation of polymerisation
- Formed by chemically joining 2 saturated bi-functional monomers, releasing a small molecule (usually water)
- There is no-double bond that opens (like in addition); the functional group of 2 monomers react together forming a new bond and water.
- Natural (DNA, cellulose, starch) synthetic (Nylon, polyester)
Nylon
- Formed when monomers with carboxylic acid and amine double functional groups react via condensation polymerisation to release water
- Repeating units are connected via amine links and can be mixed with a variety of additives to achieve different properties
- Semi-crystalline due to H-bonding between polymers
- High tensile strength, high melting point, heat resistant, UV resistant, electrically insulating
- Used to make carpets, airbags, parachutes
- Name reflects the number of carbons in the monomerPolyesters
- If 2 monomers, prefix is di
- Nylon-6 is made of 1 monomer containing 6 carbons with an amino group at one end and a carboxyl group at the other.
- Nylon-6,6 is made from 2 monomers, making it a copolymer. It has a diamine monomer with 6 carbons and a dicarboxylic acid containing 6 carbons
- Formed when monomers with carboxylic acid and alcohol double functional groups react via condensation polymerisation to release a water molecule.
- Repeating units are connected to ester link
- Most common is PET n(C10H8O4)
- Made from terephthalic acid and ethylene glycol monomer
- Polyester fibres are strong and durable, resistant to most chemicals, shrink and stretch resistant, hydrophobic, retains its shape.
- Depending on shape its either thermoplastic or themosetting
- PET is thermoplastic, colourless, lightweight. Used in plastic bottles, flexible food packaging, blister packs and trays for frozen dinner
- Can be recycled and repeatedly melted and reshaped.
Testing for unsaturation
- Bromine is added to a sample to test presence of double or triple bond and the sample is shaken
- If its an alkene/alkyne, a positive result is the disappearance of the colour è it decolourises
- If its an alkane nothing happens
- Dilute alkaline potassium permanganate (KMnO4) is added to sample
- Positive result when colour goes from purple to green and forms a brown precipitate
Test for Hydroxyl group
- A small piece is added to sample and a positive test is the effervescence of hydrogen gas
2R-OH+2Na→ 2R-O-Na+ + H2
- Oxidation testo Small volume of acidified dichromate ion (Cr2O72-) is added


















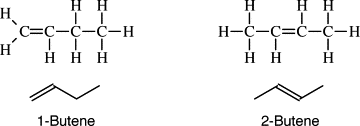










![]()


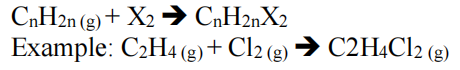





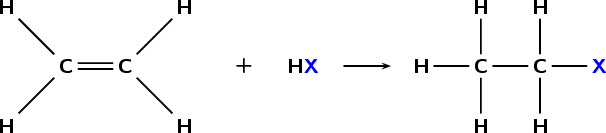







![]()
![]()